Trouble Metabolizing Fat What Diet
- Review
- Open Access
- Published:
Physiological process of fat loss
Bulletin of the National Research Centre volume 43, Article number:208 (2019) Cite this article
-
49k Accesses
-
7 Altmetric
-
Metrics details
Abstract
Background
Adipose tissue is a type of connective tissue composed of adipocytes. Recently, this tissue has been recognized as a major endocrine organ. The physiological process of fat loss occurs when fats are liberated from adipocytes into circulation to supply the needed energy. Nutrition supplements that increase fat metabolism, impair fat absorption, increase weight loss, and increase fat oxidation during exercise are known as fat burners. A good fat burner must burn the stored fats, break down the fat cells, and increase the metabolic rate. Eating thermogenic foods helps burn fats. Fat flush diet comes to repair the damage that resulted from ingested and environmental toxins stored in adipocytes.
Aim of work
This review will focus on the recent advances in fat burning supplements, fat burning foods, and fat flash diet.
Conclusion
The human body can act as a fat-burning machine by depending on low-calorie foods instead of high-calorie foods in addition to doing regular exercise, avoiding toxins and processed food, and applying any fat flush dietary program under the approval of a professional doctor. What's new in this review is that it may orient our attention to the importance of using natural fat burners in the fat burning process in an attempt to avoid medications that have many side effects by targeting other organs and it also gives an idea of the importance of increasing the brown adipose tissue content because its activation could be linked to a feeling of being full. We need further studies in this context.
Background
Adipose tissue is a loose connective tissue composed of adipocytes and is responsible for storing energy in the form of lipids; at the same time, it cushions and insulates the body (Birbrair et al. 2013). White adipose tissue (WAT) and brown adipose tissue (BAT) are the two main types of adipose tissues (Kershaw and Flier 2004). This tissue has been recognized as a major endocrine organ as it produces hormones such as leptin and resistin as well as cytokines such as TNF-α and IL-6 (Coelho et al. 2013). Moreover, the adipose tissue can affect other organs of the body systems and may lead to diseases (Cannon et al. 2018). Lipogenesis occurs in the liver and adipose tissue where carbohydrate and protein consumed in the diet can be converted to fat. The carbohydrates can be stored as glycogen in the liver and muscle and can be converted to triglycerides in the liver and transferred to adipose tissue for storage (Kersten 2001). Amino acids are used for new protein synthesis or converted to carbohydrate and fat (Wood et al. 2008). The physiological process of fat burning occurs when fats are liberated from adipocytes into circulation to supply the needed energy (Porter et al. 2009). The body needs food in acquiring the energy to feed/sustain its cells and in performing internal and external functions (Jeukendrup et al. 2016). The term "fat burner" is used to describe nutrition supplements that claimed to acutely increase fat metabolism, impair fat absorption, increase weight loss, and increase fat oxidation during exercise (Podder et al. 2011). A good fat burner must burn the stored fats, break down and mobilize the fat cells, increase the metabolic rate, and inhibit the enlargement of fat cells (Nawrot et al. 2013). Fat-burning supplements include caffeine, carnitine, green tea, conjugated linoleic acid, and chromium (Eric and Berg 2010). Fat burners contain herbal ingredients such as ephedrine, yohimbine, chitosan, and pyruvate (Nawrot et al. 2013). Most people think of eating as a way to increase fat in the body, not lose it; but there are foods that have the ability to burn fat as they are ingested (Paul 2009). Certain foods are rich in water content and thus help in the process of fat reduction (Grier 2007). Ingested and environmental toxins that were taken every day can be stored in fat cells (La Merrill et al. 2013). People who have a higher body mass index store a greater amount of toxins and they may face weight loss plateau (Jones et al. 2008). Toxins released during weight loss had the capacity to damage the mitochondria and interfere with the fat-burning hormones (Lyon et al. 2016). Fat flush diet comes to repair the damage that resulted from toxins by working on the principle of detoxifying the body, weight loss, and keeping the weight off (Gittleman 2010). This diet solves the problem that vegetarian people face by substituting animal-based proteins with plant-based proteins. The diet is also intended to help dieters who have reached a weight loss plateau resume weight loss while "flushing out" fat (Gittleman 2011). Fat burner is not meant to replace a good diet and exercise plan. The best way to help the body stimulate/metabolism and activate the fat burning abilities is to eat thermogenic foods, drink plenty of water, exercise regularly (Paul 2009). This review is an effort to summarize the role of fat burning supplements, fat burning foods, and fat flash diet to support the physiological process of fat loss.
Adipose tissue
The adipose tissue is a loose connective tissue full of adipocytes. It is responsible for storing fats in the form of triglycerides. It is found all over the body: under the skin (subcutaneous fat), packed around internal organs (visceral fat), between muscles, within the bone marrow, and in the breast tissue (Nagai et al. 2015). Men tend to store more visceral fat (around their internal organs), leading to obesity around the middle of their abdomen (belly fat) (Cannon et al. 2018). However, women tend to store more subcutaneous fat within the buttocks and thighs (Brown et al. 2017). These differences are due to the sex hormones produced by males and females (Canoy 2010).
Structure of adipose tissue
The very important God-given cellular components are found in adipose tissue in two different forms: white adipose tissue (WAT) and brown adipose tissue (BAT) (Fig. 1) (Cannon et al. 2018).
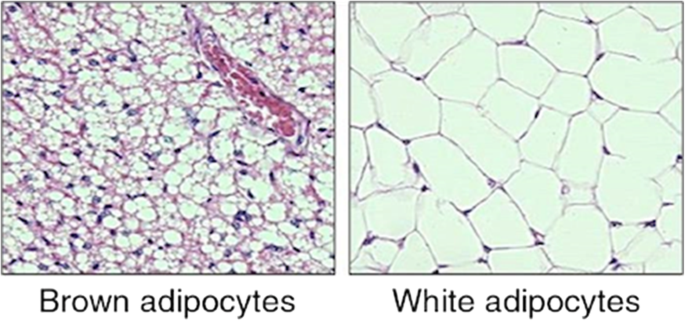
The two kinds of adipose tissue in mammals
Full size image
The presence, amount, and distribution of each vary depending on the species. WAT is the most common and is the fats that many of us complain of acquiring (Divoux et al. 2011). It is found around the waists and thighs, while BAT is mainly found around the neck areas (front and back) and supraclavicular regions (Harms et al. 2013). WAT serves three functions: heat insulation, mechanical cushion, and a source of energy (Alligier et al. 2011). BAT serves to generate body heat. It derives its color from rich vascularization and densely packed iron-containing mitochondria. It is found in various locations, depending upon the species and/or age of the mammals (Fenzl and Kiefer 2014). Instead of serving as a substrate, the lipid in BAT releases energy directly as heat, therefore it used in heat production for non-shivering thermogenesis and for utilization of excess caloric intake via diet-induced thermogenesis (Kissig et al. 2016). The mechanism of heat generation is related to the metabolism of the mitochondria. Mitochondria from BAT have a specific carrier known as uncoupling protein that transfers protons from outside to inside without subsequent production of ATP (Enerbäck 2009).
Distribution of adipose tissue
Everyone does not carry their adipose tissue in the same anatomical locations. Adipose tissue that is located in the upper body has been termed apple distribution and this distribution pattern is found more in men while adipose tissue that is accumulated in the lower body has been termed pear distribution and this distribution pattern is found more in women (Karastergiou et al. 2012).
Factors that determine fat distribution
A primary factor is genetic background, which can be seen by looking at the similarity in fat distribution within same-sex family members. As mentioned above, gender is also known to affect body fat location. After the menopause, a change towards upper body fat distribution is observed due to a decrease in hormone lipoprotein lipase (LPL) activity in the lower body region (Inagaki et al. 2016).
Risks of fat accumulation
1. Upper body fat distribution is correlated with the development of various health problems, including cardiovascular disease, hypertension, diabetes, sleep apnea syndrome, osteoarthritis, and some cancers as most of fats are packed around the internal organs (Baglioni et al. 2012).
2. Lower body fat distribution is correlated with the mechanical problems as most of fats are distributed around the hips, thighs and buttocks (Dhaliwal and Welborn 2009). Calculation of waist-to-hip-ratio is a quick test to predict the risk for complications associated with upper-body fat distribution. Women are at risk if the ratio exceeds 0.85; for men the ratio is 0.95 (Stunkard et al. 2007).
"Yo-yo" dieting "weight cycling" is one of the most important problems that correlate with the development of upper body fats. Weight cycling is defined as a repeated pattern of losing and regaining body weight. The main causes of it are losing weight rapidly, not exercising and bad eating habits (Brownell 2009). Yo-yo dieting rarely leads to success, and it results in health risks such as depression (from "failure" to manage the weight), reduced metabolism (eating too few calories forces the body into "starvation mode"), weight gain, and poor heart health (Duvernoy 2011). It leads to obesity-related problems, such as high cholesterol, high blood pressure, increased risk for heart disease, and negative blood flow to the heart leading to plaque buildup in the arteries and potentially leads to stroke or heart attacks (Mehta et al. 2014).
The process of fat deposition
Lipogenesis is the process of fat deposition that occurs in the liver and adipose tissue (Kersten 2001). Carbohydrate and protein consumed in diet can be converted to fat. The carbohydrates can be stored as glycogen in the liver and muscle and can be also converted to triglycerides in the liver and transferred to adipose tissue for storage. Amino acids are used for new protein synthesis or they can be converted to carbohydrates and fat (Wood et al. 2008).
Should we try to increase our brown fat content?
Brown fat tissue in the body can burn huge amounts of energy to generate heat, and studies in humans and animals have suggested that increasing the amount of healthy brown fat might help weight management. However, how to safely and effectively increase brown fat has been a significant challenge for researchers (Virtanen et al. 2009). The activity of this tissue changes over time: It decreases with age, just as it does in obese individuals and diabetics. Hence, ways to heat-up thermogenesis in brown fat are being sought which can be used to prevent obesity and diabetes (Kissig et al. 2016). A simple, innovative tissue-grafting strategy that increases endogenous brown fat has been developed. The method directly converted white fat to brown fat outside the body and then re-implanted it in a patient. White fat is converted to brown fat by culturing tissue fragments in media containing growth factors and other endogenous browning factors for 1 to 3 weeks to stimulate the "browning" process by measuring levels of several brown fat biomarkers, including mitochondrial activity and the brown fat protein marker UCP1. In one of the study's experiments, the researchers discovered that subcutaneous white fat in mice could be directly converted to brown fat outside the body and that the brown fat both survived and remained stable after injection into the same mouse for a long period. They then used their methods on human subcutaneous fat and were able to effectively convert it to brown fat. This suggests that it might be possible one day to attempt this approach in humans as a potential therapy to help with weight loss, control of blood glucose levels, or to prevent weight gain (Nordqvist et al. 2012). Other methods to increase brown fat include chronic cold exposure, which is uncomfortable for most people, and pharmaceuticals that can cause side effects by targeting other organs. In a study carried out on volunteers with higher brown fat levels, volunteers started shivering at lower temperatures compared to those with lower levels. These volunteers burned an extra 250 calories, a 1.8 times increase in calorie burning rate when the brown fat cells were active (Blumenfeld et al. 2018). They not only experience an increase in the heat output of brown fat in the cold as they got used to the lower temperatures but also an improvement in the control of blood sugar via insulin (Din et al. 2018). Arginine-rich foods may also stimulate brown adipose tissue growth and development through a variety of mechanisms, which is achieved by consuming more soy foods, seeds, nuts, and beans (https://nutritionfacts.org/2017/10/03/boosting-brown-fat-through-diet/).
White adipose tissue as an endocrine and secretory organ
Adipose tissue is now known to be a very important and active endocrine organ (Coelho et al. 2013). It is well established that adipocytes, play a vital role in the storage and release of energy throughout the human body. A number of different hormones are released from the adipose tissue and these are responsible for different functions within the body (Fig. 2) (Lyon et al. 2003; Trayhurn and Wood 2004; Eckel et al. 2005; Guerre and Millo 2011). Adipose tissue secretes cytokines like TNF-α, IL-6, and IL-1β, which are involved in sending messages between cells (Coelho et al. 2013).
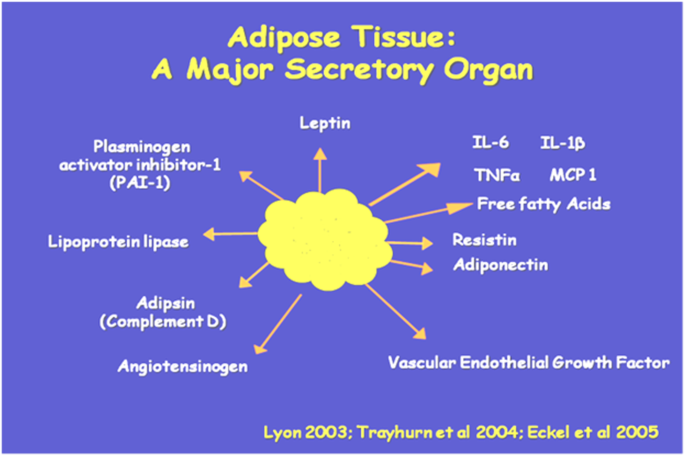
Adipose tissue as an endocrine and secretory organ
Full size image
What happens when fat cell is burned?
Most people really do not know how the fat cells work, how the fat burning process takes place, or where the fat goes when it is burned. It is actually quite a complex physiological process, but many researchers and experts explained it as simply as possible. When the body loses fat, the fat cell does not go anywhere or move into the muscle cell to be burned. The fat cell itself stays right where it was under the skin in thighs, hips, arms, etc., and on top of the muscles, which is why it is difficult to see muscle "definition" when the body fat is high (Porter et al. 2009). Fat is stored inside the fat cell in the form of triaglycerol. The fat is not burned right there in the fat cell; it must be liberated from the fat cell through somewhat complex hormonal/enzymatic pathways. When stimulated to do so, the fat cell simply releases triaglycerol into the bloodstream as free fatty acids (FFA's), and they are transported through the blood to the tissues where the energy is needed (Manore et al. 2011). By lipolysis, each molecule of triaglycerol splits into glycerol and three fatty acids. The reaction catalyzed by hormone-sensitive lipase (HSL). The stored fat gets released into the bloodstream as FFA's and they are shuttled off to the muscles where the energy is needed. As blood flow increases to the active muscles, more FFA's are delivered to the muscles that need them. FFA's get inside the mitochondria by LPL and this is where the FFA's go to be burned. When the FFA's are released from the fat cell, the latter shrinks and that is the reason for the leaner look when the body loses fat because the fat cell is now smaller (Fig. 3) (Turcotte 2000). The scientists concluded that "we don't actually "lose" fat cells, we "empty out" fat cells," our body fat is basically just a reserve source of energy and fat cells are like the storage tanks. Unlike a gas tank in the car that is fixed in size, fat cells can expand or shrink in size depending on how "filled" they are (Robergs and Keteyian 2013). People remain thinner even after having more energy food/fats because of more than one reason (https://www.sbs.com.au/food/health/article/2018/06/12/why-do-slim-people-who-eat-lot-never-seem-put-weight):
- 1.
Not only because they have a faster metabolism than everyone else as the old adage goes, but also because of their calculated total calorie intake.
- 2.
They slept 6–8 h a night, drank little or no soft drinks (avoiding excessive sugar contents), rarely ate out (processed foods are kept to a minimum), ate meals sitting down (people who ate a meal standing up, ate twice as much after they finished consuming the food, therefore they considered the food to be a snack, not a meal), do not really snack a lot and also built an eating and exercise routine into their lives (meals were eaten at regular times during the day).
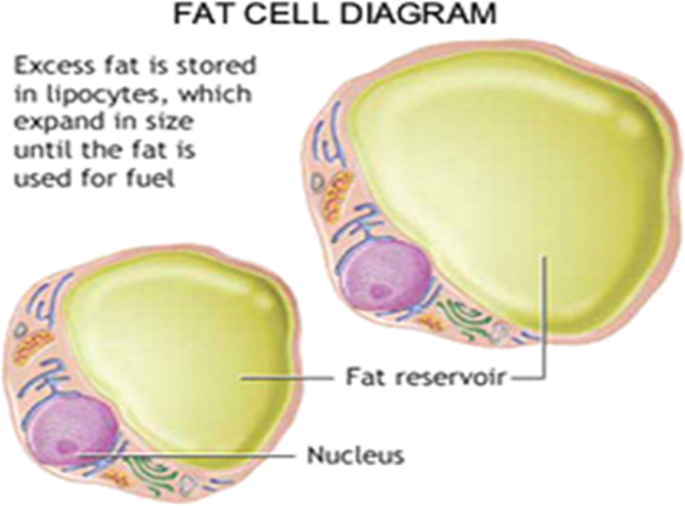
The incredible shrinking of fat cell
Full size image
What could go wrong with adipose tissue?
Too much and too little adipose tissue can cause severe health implications. More commonly, too much adipose tissue leads to obesity, mainly from too much visceral fat. Obesity leads to a number of serious health problems. It increases the risk of developing type 2 diabetes as it causes the body to become resistant to insulin. This resistance results in high levels of blood sugar which is bad for health. Obesity also increases the chance of developing high blood pressure, high cholesterol levels, and an increased tendency for blood to clot leading to risk of heart attacks and stroke (Anderson et al. 2017). A lack of adipose tissue, lipodystrophy, can also cause similar problems and is seen with increasing frequency as a result of medication used to treat HIV/AIDS. In eating disorders, such as anorexia nervosa, the patients do not eat enough food to maintain their adipose tissue levels. This means that they can lose a dangerous amount of body weight (Kershaw and Flier 2004). Anyone who is overweight and/or obese has some kind of insulin resistance, but diabetes only develops in those individuals who lack sufficient insulin secretion to match the degree of insulin resistance. Insulin in those people may be high, yet it is not enough to normalize the level of glycemia (Al-Goblan et al. 2014). In order to develop insulin resistance and obesity, thereby causing type 2 diabetes, β-cells should not be able to compensate fully for decreased insulin sensitivity. The non-esterified fatty acids (NEFAs) that are secreted from the adipose tissue in obese people may lead to the hypothesis that insulin resistance and β-cell dysfunction are most likely linked (Kahn et al. 2016).
Fat burners
When we talk about fat loss, much of the information available can be confusing. Commonly, too many calories are mistakenly cut to really low levels to accelerate fat loss, almost certain to leave the plateau where further fat loss is difficult. Rapid caloric reduction will cause a decrease in muscle mass and slow basal metabolism. The body should be stimulated by certain fat burner to liberate fat, without fat cells resisting are being broken down as fuel (Paul 2009). The term "fat burner" is used to describe nutrition supplements that claimed to acutely increase fat metabolism or energy expenditure, impair fat absorption, increase weight loss, increase fat oxidation during exercise, or somehow cause long-term adaptations that promote fat metabolism (Jeukendrup and Randell 2014). These supplements contain a number of ingredients, each with its own proposed mechanism of action (Podder et al. 2011) and it is often claimed that the combination of these substances will have additive effects (Lenz et al. 2013). A good fat burner must burn the stored fats for energy, break down and mobilize the fat cells, and increase the metabolic rate to burn stored fats and inhibit fat cells from enlarging (Nawrot et al. 2013).
Fat-burning supplements
The list of supplements that claimed to increase or improve fat metabolism is long. The most popular supplements include caffeine, carnitine, green tea, conjugated linoleic acid, and chromium (Westerterp-Plantenga et al. 2005; Westerterp-Plantenga 2010; Kennedy et al. 2011; Reuter and Evans 2012; Onakpoya et al. 2013). Fat burners contain herbal ingredients such as ephedrine, yohimbine, chitosan, and pyruvate (Eric and Berg 2010), all of which operate on the principle of thermogenesis to increase energy, stimulate the metabolism, and/or suppress the appetite (Shekelle et al. 2003a; Cohen et al. 2016; Rios-Hoyo et al. 2016; Onakpoya et al. 2014).
Classes of supplements
In this review, the evidence for some of these supplements is briefly summarized. Based on the available literature, caffeine and green tea have data to back up its fat metabolism-enhancing properties. For many other supplements, although some show some promise, evidence is lacking. The list of supplements is industry-driven and is likely to grow at a rate that is not matched by a similar increase in scientific underpinning.
Energy enhancers
1. Caffeine is found in coffee, tea, soft drinks, cocoa, and cola nut. It has been most widely used in fat-loss products (Greer et al. 2000). Caffeine is not only an energy enhancer but it is also becoming a popular fat loss supplement and workout performance booster (Schwenk et al. 2003). Many published studies are documenting its effectiveness at weight loss and reducing body fat (Acheson et al. 2012; Dulloo et al. 2009). These studies have shown that caffeine is capable of increasing the release of stored fat, as well as the rate at which calories are burned. The effects of caffeine are best realized when used in combination with other supplements. The most popular combinations have been the ephedrine/caffeine/yohimbine in ECY stack and ephedrine/caffeine/aspirin in ECA stack (Paul et al. 2011; Dulloo et al. 2015). Caffeine promotes fat loss at two major sites: fat cells and muscle cells. The action of caffeine at the fat cell appears to be supportive of the fat loss signal generated by neurotransmitters and drugs that stimulate β-adrenergic receptors (Klein et al. 2006). These receptors are stimulated by adrenalin and similar chemicals. The level of adrenalin-like drugs, hormones, and neurotransmitters released at any point in time is called the "sympathetic tone." The sympathetic tone may rise quite high during a "fight-or-flight" response or it may be low during periods of rest or sleep (Arciero et al. 2007). At rest and without the addition of caffeine, there is not much fat release or increase in calorie burning because of the effect of a competing class of adrenergic receptors, A2-adrenergic receptors (Belza et al. 2014). While β-receptors promote fat loss and increase calorie burning, the A2-receptors do the opposite (Hursel et al. 2016).
2. Green tea extract contains catechin epigallocatechin-3-gallate (EGCG), the active ingredient (Cabrera et al. 2006) that may help boost the metabolic rate by stimulating the metabolism, helping the body to burn more calories, and may possibly lead to weight loss (Lambert et al. 2007). It was discovered that EGCG inhibits catechol-O-methyltransferase, an enzyme that breaks down norepinephrine. The higher levels of norepinephrine in the body enhance the overall rate of fat loss by stimulating the release of fatty acids from fat cells into the bloodstream for burning as fuel (Johnson et al. 2012). Researchers examined the effects of green tea on weight loss in obese men and women. Participants followed a diet plan with green tea or a placebo for 12 weeks. Energy expenditure and fat oxidation, or fat burning, were measured at the beginning of the study and during weeks 4, 8, and 12. Scientists observed that the green tea group lost more body fat compared to the placebo group (Hofman et al. 2008; Ha and Zemel 2011).
Protein and amino acids products
1. Whey Protein constitutes approximately 20% of the total protein found in milk. Protein supplementation is used by athletes to promote positive nitrogen balance throughout the day without dramatically increasing caloric intake (Demling 2009). Specifically, it is reported that whey protein may help build muscles, increase strength, control appetite, aid in weight loss, improve endurance, and boost energy levels (Boirie et al. 2011).
2. Casein is a protein derived from milk products. It is used primarily by athletes to increase muscle mass and strength, control appetite, aid in weight loss, improve endurance, and boost energy levels. It provides all of the amino acids necessary for growth (Delbeke et al. 2002). Casein protein forms a gel in the stomach, which allows it to be digested more slowly so the peptides/amino acids are absorbed steadily over a long period of time, unlike whey protein, which is absorbed very quickly (Sulcová et al. 2005).
Testosterone enhancers
1. 7-Keto-DHE is produced by metabolism of the dehydroepiandrosterone (DHEA) prohormone and is not directly converted to testosterone or estrogen and declines with age. Supplementation with 7-Keto may help increase the metabolic rate, accelerate weight loss, and help burn fat (Bobyleva et al. 2007; Leonard et al. 2014). When used topically, skin lotion product, 7-Keto caused long-lasting changes in the body's levels of testosterone, epitestosterone, estradiol, and other steroid hormones (Lardy et al. 2007; Zenk et al. 2012).7-Keto might improve protein synthesis to preserve more lean muscle on a caloric deficit and support the weight loss efforts when combined with a fat loss nutrition and exercise plan (Bucci 2008). 7-Keto demonstrates thermogenic activity through the activation of three enzymes: glycerol-3-phosphate dehydrogenase, malic enzyme, and fatty acyl CoA oxidase (Cimolai et al. 2011). These enzyme activations drive energy-producing substrates in a direction of less efficient ATP production relative to heat production. The enzymes also promote the utilization of fat stores for energy and heat production (Haller et al. 2012).
2. Yohimbe comes from the bark of a particular African tree. It may be burn off stubborn body fat (Rao et al. 2017). Yohimbe is most often promoted in dietary supplements as effective in increasing muscle mass by boosting testosterone levels, accelerating weight loss, and increasing energy levels (Zahorska et al. 2011). It is famous by enhancing blood flow and makes the oxidation of fatty acids easier (Gades et al. 2002; Ylitalo et al. 2012). Yohimbine acts on the adrenergic receptor system of fat cells, which regulate thermogenesis. The beta-subunits of the adrenergic receptors (targets of ephedrine) can be seen as stimulatory for fat loss as they increase the activity of the enzyme adenyl cyclase and subsequently cAMP levels (Manore 2015). The alpha-subunits are more suppressive of fat metabolism, in which their activation reduces activity of adenyl cyclase and reduces cAMP levels (specifically alpha-2) (Carmen and Víctor 2006). Yohimbine is a selective alpha-2 adrenergic receptor antagonist as it has a 45-fold higher affinity for the alpha-2 subunit than it does for the alpha-1 subunit, which inhibits activation of the suppressive set of receptors and preserves adenyl cyclase activity and the effects mediated via the beta receptors (Lalchandani et al. 2002). Yohimbine itself can potentially induce fat loss indirectly via the release of adrenaline which is an activator of beta-adrenergic receptors (MacDonald et al. 1997). However, this increase of adrenaline may fade with time reaching statistical insignificance 2 weeks after daily ingestion. Increases in plasma free fatty acids and the density of alpha-2 adrenergic receptors remain similar at both time points, suggesting that yohimbine selectively loses the spike in adrenaline but not direct receptor fat burning effects (Reiner et al. 2010).
Lean body enhancers
1. Chitosan is a non-digestible fiber extracted from the shells of crabs, lobsters, and other crustaceans. It is prepared in supplement form for products as Chitosan-C and Chitorich (Galitzky et al. 2012). It is an effective fat binder; it enters the body, binds to the fat in the food, and keeps it from being absorbed by the body (Zenk et al. 2012). There are two downsides to this method. First, fat blockers can prevent the body from absorbing nutrients it needs. Second, this bound fat still needs to leave the body, which it often does in the form of stomach pain, unpleasant anal leakage, and diarrhea. Chitosan is recommended for reducing lowering cholesterol levels and promoting weight loss (Pooyandjoo et al. 2016; Vincent et al. 2003).
2. L-Carnitine is a catalyst synthesized from amino acids and required for the transport of fatty acids from the bloodstream into the mitochondria during fats breakdown to generate metabolic energy for maintaining a healthy body weight. It is widely available as a nutritional supplement (Anton et al. 2008).
3. Chromium is a trace element, which can increase insulin efficiency. It reduces insulin resistance, and glucose is diverted towards muscle rather than into fat storage (Tian et al. 2016). It builds muscle at the expense of body fat gains (Fomous et al. 2002). Chromium supplementation led to reduced cravings for fat, not carbohydrates (Shekelle et al. 2003b).
4. Ephedrine is a sympathomimetic amine, derived from various plants in the genus Ephedra, commonly used as a powerful stimulant, weight loss supplement, and appetite suppressant (Clapham and Arch 2007). It helps the body lose fat by way of raising the body's core temperature through the process of thermogenesis, thereby increasing the metabolic rate speed and burning more calories (Rios-Hoyo et al. 2016; Uckoo et al. 2011).
5. Synephrine is an alkaloid, derived primarily from the immature fruit of Citrus aurantium, and is commonly used in weight loss. It has gained significant popularity as an alternative to ephedrine but it is safer and effective than ephedrine (Bredsdorff et al. 2015).
6. Raspberry ketone is a natural phenolic compound of red raspberries, cranberries, and blackberries (Cotten et al. 2017). In mice, raspberry ketone has been shown to prevent high-fat-diet-induced elevations in body weight when given in very high doses, up to 2% of body weight (Park 2010). However, no effects on body weight of rats were observed with doses up to 200 times greater than the estimated intake in humans (Ivy 2004). The high-dose effect is reported to stem from the alteration of lipid metabolism, increasing norepinephrine-induced lipolysis (Onakpoya et al. 2014).
7. Pyruvate is the salt of pyruvic acid, found in most dietary supplements combined with a mineral such as calcium or magnesium to improve stability (Whingham et al. 2007). It found to enhance weight loss, decrease appetite and fatigue, as well as increase energy levels, exercise endurance, and muscle glycogen stores (Onakpoya et al. 2012).
8. Conjugated Linoleic Acid (CLA) has been found by researchers to encourage fat breakdown (Millan et al. 2012). CLA transports dietary fat into cells to be burned for energy or used to build muscle (Brenot et al. 2015).
Appetite suppressants
They are manipulating the body's chemicals and hormones to trick the brain into feeling full. Suppressants that take away general hunger are called noradrenergic drugs (Gray et al. 2016). These drugs are often cousins of amphetamines and work by triggering fight-or-flight hormones than interrupt the body's hunger signals to the brain (Smith et al. 2014). The other class of suppressants works by manipulating serotonin reuptake to prevent a sense of the need to eat more (Whelan et al. 2010). For example, Hoodia gordonii, a plant in the South African desert, was originally used by hunters to ward off hunger and regulate thirst on long hunting expeditions. This splendid plant can help to bide the time between meals (Madgula et al. 2008). A purified extract of Hoodia, known as P57, was injected in an in vivo study directly into the brains of rats and easily broken down by the liver (Spadafranca et al. 2013). However, an in vitro study was found that P57 was generally not inhibited metabolically by human liver enzymes and has a relatively high secretion rate (Fu et al. 2016).
Miscellaneous
1. Carb Blockers are composed of the extract of Phaseolus vulgaris, the botanical name for kidney bean and often mixed with other ingredients such as chromium, vanadium, and fenugreek (Kelly et al. 2009). They are preventing the enzyme alpha-amylase which is produced in saliva from binding with starches and break down the carbohydrates into molecules that the body will absorb (Mussolino et al. 2010).
2. Thyroid regulators, such as guggul extract, are an ingredient which is found to improve thyroid functioning to produce more thyroid hormones and increase fat metabolism. These supplements may keep the basal metabolic rate (BMR) at higher levels to burn and lose more weight (Wilson et al. 2011).
Fat burning supplements safety
Some authorities claim that these supplements can safely be used in small amounts and they can be effective at jump-starting weight loss. But the means the drugs employ to do so involve manipulating the body's natural processes, sometimes to a severe degree. Side effects are expected and ranged from uncomfortable to fatal (Smith 2016). Anyone with any sort of pre-existing heart, hormonal, or digestive condition should seriously consider avoiding fat burning supplements. For other people, it remains a personal choice, but one that should be made with a certain degree of wariness (Berdanier et al. 2000). Most fat burner supplements often contain questionable ingredients and increase risk for an array of serious consequences, including heart palpitations, seizures, psychosis, severe anxiety, distress, and mood swings (Wardlaw et al. 2002).
Macronutrients
There are three macro-nutrients that can be present in food: fat, protein, and carbohydrate. One gram of protein yields 4 calories, 1 g of carbohydrate yields 4 calories, and 1 g of fat yields 9 calories. So basically, fats can provide double calories to the body compared to the other two nutrient units, but this does not mean that fats are bad, they are not (Montama et al. 2010). Many healthy fats are essential for the harmonious functioning of the body and foods that provide these fats need to be an integral part of the diet (Schmitt et al. 2015). The body needs food in acquiring the energy to feed/sustain its cells and in performing internal and external functions. The body also needs to expend energy in order to digest foods to get energy from them, so a small percentage of old fuel is burnt off in the process of acquiring new fuel. The harder the food is to digest the more energy is expended to digest it (Jeukendrup et al. 2016).
Fats: the good, the bad, and the ugly
Fat is an important nutrient for health and plays many different roles in the body. It supplies the body with energy, helps the body absorb vitamins A, D, E, and K, and helps the body grow and develop (Vanhala et al. 2012).
The good: unsaturated fats
They are the shining stars of the fat's world. They found to lower cholesterol levels and reduce the risk of heart disease. They are divided into monounsaturated fat, which can be found in avocados, nuts, and vegetable oils (Hooper et al. 2015), and polyunsaturated fat, omega-3, and omega-6, which can be found in soft margarines, fatty fish, fish oils, nuts and seeds, and vegetable oils (Schneider et al. 2016).
The bad: saturated fats
They can be found to raise LDL or "bad" cholesterol levels and increases the risk of heart disease (Leonard 2008). They are found in beef and veal meat, chicken meat, and dairy products (Kim et al. 2014).
The ugly: trans fats
They are made from a chemical process known as partial hydrogenation; this is when liquid oil is made into solid fat. Trans fats have been shown to raise bad cholesterol (LDL) levels and lower good cholesterol (HDL), increasing the risks for heart disease (Prentice 2017). They are found in hard margarines, shortening, cakes, cookies, crackers, croissants, doughnuts, muffins, pastries, and other snack foods (Carlsen et al. 2011).
How does fat burn fat?
Although fat contains more calories than protein or carbohydrates, the secret is in what fats actually do when they enter the body that makes the difference. Saturated and trans fats, especially when combined with high carbs will pile on the pounds. There is a two-step process involved in fat burning when we are on a fat loss plan: First step, cutting down on carbohydrates will release the body from the grip of the "fat storing" monster. Controlling carbs allow the body to return to its natural ability to burn fat. As long as the carbs intake is controlled, the calories from fat are immediately used for energy which means they would not be stored. The second step, involves the consumed fats within the body to generate energy. Trans fats, found in pre-packaged foods and baked goods, and anything deep fried should be avoided. Anything with hydrogenated or partially hydrogenated vegetable oil is a pound packer (Vanhala et al. 2012; Bes-Rastrollo et al. 2017).
Fat-burning natural foods, rich in good fats
Good fats help in burning body fat, not to feel hungry, enhance metabolism, and stimulate certain hormones that have many functions within the body. Fats only need to be 10–30% of the diet if we are trying to lose weight or build muscle. There are certain foods that are better than others for fat burning: Avocados, is rich in oleic acid and beta-sitosterol (monounsaturated fats), it helps in fat burning and lowers LDL cholesterol and triglycerides (Schneider et al. 2016). Nuts (cashews, pecans, almonds, walnuts, peanuts) (Harris 2011; Parra et al. 2008), fatty fish (mackerel, salmon, tuna), fish oils, and vegetable oils (canola, peanut, corn, safflower, flaxseed, soybean, sesame, sunflower) are rich in omega-3 and omega-6 (polyunsaturated fat) which increase the body metabolism and leading burn fats (Naber et al. 2014). Omega-3 fats help burn fat by enhancing the body response to leptin that signals the brain to suppress appetite and eat less for maintaining weight loss. Leptin stimulation reduces the activity of neuropeptide Y, a neurotransmitter that can trigger the hunger reflex (Holm 2009) and this in turn increase the metabolism by enhancing the thyroid output (King 2012). Palm oil, coconut oil, and cow butter contain medium chain triglycerides (MCTs), saturated fats, with an unusual chemical structure that can be digested easily. They contain fewer calories than other fats and they are absorbed and used directly for energy. Foods rich in MCTs suppress the appetite and help lose body fat (Naber et al. 2015).
How does protein burn fat?
All literatures, articles, and newsletters refer that the five mechanisms of how proteins burn fats were achieved by consuming protein-rich meals, which stimulate glucagon and growth hormone release and rich also in nitrogen, boost metabolism, suppress appetite, and get us to feel full (Karst et al. 2010; Gannon and Nuttall 2011) (https://www.johnsonfitness.com/blog/5-ways-to-naturally-boost-hgh/). We wrote in the next section some examples of these proteins. The following mechanisms are through which protein may assist the body in burning fat more effectively.
Mechanism 1: Stimulates glucagon release
Protein stimulates glucagon release from the pancreas which is insulin's antagonist hormone. By keeping insulin production low, the body can access and utilize fat as a fuel source more effectively. Glucagon stimulates the liver breakdown of glycogen to glucose and stimulates the gluconeogenesis in the liver by increasing the uptake of amino acids. It has also fat-burning effects by activating an enzyme that stimulates cyclic AMP which activates HSL (https://www.livestrong.com/article/313163-what-foods-are-high-in-nitrogen/).
Mechanism 2: Stimulates growth hormone release
Protein stimulates growth hormone (GH) release from the anterior pituitary. GH may indirectly promote fat loss. It acts directly on the fat cells and stimulates the release of fatty acids and glycerol into the blood stream. A particular amino acid, glutamine, has been shown to dramatically boost growth hormone release in the body, which then may promote greater fat burning (Guo et al. 2016).
Mechanism 3: Increases protein synthesis
Protein provides the building blocks of body tissues and regular consumption of it, i.e., as a component of 5 or 6 small meals a day make the body stay in a positive nitrogen balance then elevate metabolism, which promotes greater energy expenditure and therefore greater fat burning (Rajamohan et al. 2016).
Mechanism 4: Boosts metabolism
Not only does protein promote greater energy expenditure by maintaining an elevated metabolic rate but it also boosts the metabolism because it requires more energy to be digested compared to the other macronutrients, carbohydrate, and fat. As a result, the thermic effect of food (TEF), which means the amount of energy expended through the process of digestion increases, which increases the overall amount of calories the body burns during the day (Banni 2012).
Mechanism 5: Suppresses appetite
Protein has powerful appetite suppressing effects, especially compared to the other macronutrients. Its appetite-suppressing qualities come from the fact that protein stimulates the release of cholecystokinin (CCK) from the stomach cells. This hormone then travels through the bloodstream to the hypothalamus in the brain where it tells the brain that the stomach is full (Whingham et al. 2007).
Fat-burning foods, rich in proteins
From the aforementioned 5 mechanisms, it is easy to conclude why protein can help promote fat burning in the body. It can help in fat burning by simply making an effort to add a small portion of protein, from a variety of protein sources to each meal. Protein, foliate, and vitamin D are found in red meat (beef, veal, pork), skinless turkey, and chicken. Proteins are not only more complex to digest and assimilate but they also require more energy to be stored as fats, so, they help to feel full and help the body in fat loss (Coyle and Patrick 2013).
Dairy products provide whey protein and casein that build muscle, control appetite, and aid in weight loss (Boirie et al. 2011; Delbeke et al. 2002; Sulcová et al. 2005). They contain CLA that works to lower the triglycerides and cholesterol leading to upregulate the body metabolism (Leonard 2008; Kim et al. 2014; Yang et al. 2004). The process of converting dairy down into lactic acid causes the body to utilize the energy stored in fat. Low-fat dairy products such as cheese, milk, and yogurt contain calcium and complex carbohydrates which work to kick metabolism into action and burn fat (Villarroel et al. 2014). Peanut butter provides protein, vitamins B3 and E, magnesium, cortisol, foliate, dietary fiber, and arginine all of which increase protein synthesis, boost metabolism, and help in fat burning (Christensen et al. 2009). Eggs are rich in satiating protein. Eggs for breakfast can boost weight loss plan more than a carbohydrate-rich breakfast (Soerensen et al. 2014).
Micronutrients
Calcium
Several studies have correlated higher calcium intakes with lower body weight or less weight gain over time (Parikh et al. 2003; Yanovski et al. 2009; Chen et al. 2012). Two explanations have been proposed. First, high-calcium intakes might reduce calcium concentrations in fat cells by decreasing the production of parathyroid hormone and the active form of vitamin D. Decreased intracellular calcium concentrations, in turn, might increase fat breakdown and discourage fat accumulation in these cells (Earthman et al. 2012). Second, calcium from food or supplements might bind to small amounts of dietary fat in the digestive tract and prevent absorption of this fat (Mallard et al. 2016; Pathak et al. 2014).
Vitamin D
Observational studies indicate that greater body weights are associated with lower vitamin D status, and obese individuals frequently have marginal or deficient circulating levels of vitamin D (Lim et al. 2012). Although obesity does not affect the skin's capacity to synthesize vitamin D, greater amounts of subcutaneous fat sequester more of the vitamin and alter its release into the circulation. Nevertheless, the association between vitamin D and obesity raises the question of whether increasing vitamin D concentrations might reduce body weight (Gittleman 2011; Young et al. 2010).
Other fat-burning foods
1. Fruits, although all fruits are strong healthy food, the fact is that only some have fat-burning properties. The best choices include citrus fruits; the low-glycemic fruits (lemons, limes, oranges, tangerines, and grapefruits), grapes, cherries, and kiwi fruits. These fruits contain vitamin C, which not only works to dilute fat and cholesterol by its acidity but also helps release the fat cells. Apples and berries, especially raspberries, are the most pectin-rich fruit which limits the ability to absorb fat. Another choice is peaches, pears, plums, strawberries, and pomegranates. They are rich in vitamins and minerals, high in water content, and have low glycemic index. They found to improve body metabolism and reduce bad cholesterol (Denker et al. 2012). Bananas and mangoes make for excellent snack foods as well as breakfast foods. Berries are extremely high in B vitamins that stimulate the thyroid hormone and boost metabolism. It is advised to eat the fruits whole for added fiber and increased the feeling of fullness (Gittleman 2012). Grapefruit has been an integral part of many diets. Its fat-burning mechanism is due to the high-fiber content that is known to burn more calories during the digestive process than calories in the grapefruit itself. Additionally, grapefruit's low sodium and 90% water composition aid in flushing excess fluids from the body, which diminishes the appearance of cellulite. Grapefruit pills were found to improve insulin resistance compared to its juice (Terry 2011).
2. Vegetables have a low-calorie profile while containing essential minerals and vitamin that improve the metabolism of the body, except for certain calorie-rich vegetables like potatoes and sweet potatoes. Potatoes were preferred to be cooked with the outer skin because it is a good source of insoluble fibers (Li 2018). Veggies like broccoli, spinach, artichoke, peas, cauliflower, cabbage, and carrots are excellent sources of minerals and have low calories that offer fat burning. They are rich in fiber, which delays hunger (Biesiekierski 2017). Cucumbers are high in sulfur and silicone, both of which help the body rid itself of fat content. Beets are rich in iron, potassium, magnesium, and fiber. They enrich the blood and aid in liver function, thus helping to rid the body of fat through elimination (Whitehead et al. 2014). Onions and garlic also make great fat burners. The best way to cook veggies would be to boil them or stir fry them with healthy oils like olive oil, sunflower oil, Soybean oil or sesame oil (Julkunen et al. 2012).
3. Grains and Seeds are rich in fibers which can control the blood sugar. Oats are rich in fiber, especially, insoluble β-glucan which is found in researches to stabilize the blood sugar of type II diabetics better than other types of fiber and improves metabolism (Ramdath et al. 2016). Oats also are digested slowly, keeping insulin production down. It is advised to eat one bowl of oatmeal at breakfast (Mudryj et al. 2014; Serna-Saldivar 2015). Lentils and other legumes are not only filling and nutritious but are also low on calories and rich in plant proteins/amino acids. They can balance copper and zinc which support thyroid function and boost metabolism (Whiting et al. 2014). They are excellent sources of dietary fiber and are known to lower the bad cholesterol and thus contribute to heart health. The best way to eat legumes would be to eat the whole grains (Earthman et al. 2012). Not only is flax oil rich in omega-3 but it also is found to lower cholesterol (van Avesaat et al. 2016).
4. Thermogenic foods, are foods that help burn fat by heating up the body (Pathak et al. 2014). Capsaicin, a well-known thermogenic compound found in chili peppers, jalapenos, and ginger, works to heat up the body, speed up metabolism, and burn fats (Rhoades and Tanner 2003).
Water
It would not count as food because it has no calories. Water helps improve the overall metabolism of the body and thus helps burn fat. And of course, water helps flush out toxins and thus improves the capacity of the body to stay healthy (Gittleman 2011). Many studies have shown that extra water intake, especially up to 500 ml at mealtime, was conducive to weight loss (Stookey et al. 2008; Dennis et al. 2010; Dubnov-Raz et al. 2011; Muckelbauer et al. 2013; Vij and Joshi 2013). Certain foods are rich in their water content and thus help in the process of fat reduction and feeling full quickly, for example are watermelons, cantaloupes, cucumbers, snake gourd, papaya, and chard (Rosenberg et al. 2010; Naz et al. 2014). The benefit of eating these water-rich foods is that they supply minerals along with water and hence do not cause "water intoxication" which can result from drinking too much water while not balancing out the minerals especially during workouts (Grier 2007; Farrell et al. 2013).
The link between weight gain, weight loss, and toxins
The ingested and the environmental toxins that were taken every day can be stored in fat cells. People who have a higher body mass index store a greater amount of toxins and they may face weight loss plateau (Jones et al. 2008). Toxins released during weight loss had the capacity to damage the fat-burning mitochondria and interfere with the thyroid hormones and their receptor sites, interfere with enzymes, and interfere with leptin signals to hunger reflex. Thus, if we lose a lot of weight, we release into the body's circulatory system a greater amount of stored toxins; the damage was significant enough to lower the body's ability to burn calories (Bland et al. 2006). A number of studies have been found that a decreased metabolic rate is in response to the presence of toxins affecting the thyroid hormones and the rate at which the liver excretes them (Hsueh et al. 2013; Imbeault et al. 2016).
Fat flush diet (detox diet)
Fat flush is a low-carbohydrate eating plan devised with a focus on weight loss while detoxing the liver and lymphatic systems to enhance overall health. In addition to limiting carbohydrates, it recommends eating fat-burning fats, high-fiber vegetables and fruit, clean protein, and thermogenic foods and supplements (Gittleman 2012). Caloric intake on the fat flush plan ranges from 1100 to 1600 calories per day, which is in line with the nutrition recommendations for weight loss (Klein and Kiat 2015).
The fat flush plan
It has three phases (2 weeks each), the first phase is the most restrictive, limiting dieters to 1100 to 1200 calories daily and aims to "lose bloat," or water retention, and include some weight loss. During this phase, margarine, sugar, oils (except flaxseed oil), grains, bread, cereal, starchy vegetables, dairy products, and some spices are restricted. During the second phase, calories are increased from 1200 to 1500 calories daily. It includes the same food that is in the first but with the addition of butternut, sweet potato, fresh or frozen peas, brown rice, and carrots once weekly. This phase continues until reaching the needed weight. The last phase is to maintain weight loss and entail 1500 or more calories daily. Certain foods that were eliminated in phase 1 are reintroduced back such as some starchy carbohydrates, dairy, and gluten-free grains (Gittleman 2010).
The function of fat flush plan
It aims to cleanse the liver, improve wellness, and produce weight loss. The plan is based on a "cleansing combination" of essential fats, proteins, and healthy carbohydrates. Eating in this way enhances the liver's ability to function optimally, accelerates weight loss, and improves health. The diet is also intended to help dieters who have reached a weight loss plateau resume weight loss while "flushing out" fat (Gittleman 2011). An expert opinion is that the elimination of all margarine, fats, oil, sugar, bread, grains, high-carbohydrate vegetables, and dairy products can be difficult for some people because they found the remaining food list so restrictive. Fat flush plan is incompatible with vegetarian diet because of the importance of eating lean protein from animal sources, which they cannot do; so vegetarians face difficulty in following this diet. The plant-based protein could be a substitute animal-based protein for vegetarians. Protein found in soybean and legumes is considered as an acceptable protein substitute on the Fat flush plan. The lacto-ovo vegetarians consume eggs, light yogurt, and light cheeses as a source of protein (Picco 2012).
Conclusion
We can turn our body into fat-burning machine by including low-calorie foods instead of high-calorie foods in our diet. The fat burning supplements are not alone to burn fats alone. Without a proper diet and regular exercise, we cannot reach the needed goal. If we decide to start any fat flush dietary program, we should seek approval from the doctor prior to starting. To avoid toxins which delay the burning process, we should eat organic foods as much as we can, avoid processed foods, and use natural product to be away from chemicals, additives, or preservatives.
Recommendation
Too much fats increase the risk of diabetes with the alarming complications of cardiovascular disorders. Modification of an unhealthy diet, bad eating habits, and lifestyle factors should remain the cornerstone in managing body fats. New kinds of natural foods should be added in daily meals to improve fat burning process to avoid health complications. Scientific efforts must certainly be more oriented to discover how we should try to increase our brown fat cells to help in fat burning.
Availability of data and materials
Not applicable.
Abbreviations
- Acetyl CoA:
-
Acetyl coenzyme A
- ATP:
-
Adenosine triphosphate
- BAT:
-
Brown adipose tissue
- BMR:
-
Basal metabolic rate
- CCK:
-
Cholecystokinin
- CLA:
-
Conjugated linoleic acid
- DHEA:
-
Dehydroepiandrosterone
- ECA:
-
Ephedrine/caffeine/aspirin
- ECY:
-
Ephedrine/caffeine/yohimbine
- EGCG:
-
Epigallocatchin-3-gallate
- FFA's:
-
Free fatty acids
- GH:
-
Growth hormone
- HDL:
-
High-density lipoprotein
- HIV/AIDV:
-
Human immunodeficiency virus
- HSL:
-
Hormone sensitive lipase
- IL-1β:
-
Interleukin-1 beta
- IL-6:
-
Interleukin-6
- LDL:
-
Low-density lipoprotein
- LPL:
-
Lipoprotein lipase
- MCTs:
-
Medium chain triglycerides
- TEF:
-
Thermic effect of food
- TNF-α:
-
Tumor necrosis factor-alpha
- WAT:
-
White adipose tissue
References
-
Acheson KJ, Zahorska MB, Pittet PY, Jéquier SD (2012) Metabolic effects of caffeine in humans: lipid oxidation or futile cycling? Am J Clin Nutr 33(5):989–997
Article Google Scholar
-
Al-Goblan AS, Al-Alfi MA, Khan MZ (2014) Mechanism linking diabetes mellitus and obesity. Dia Metab Syndr Obes 7:587–591. https://doi.org/10.2147/DMSO.S67400
Article Google Scholar
-
Alligier M, Meugnier E, Debard C, Scoazec J (2011) Subcutaneous adipose tissue remoduling during the initial phase of weight gain induced by overfeeding in human. J Clin Endocrinol Metab 10(15):2314–2327
Google Scholar
-
Anderson G, James W, Konz E (2017) Obesity and disease management: effects of weight loss on comorbid conditions. J Am Med Assoc. 16:23–36
Google Scholar
-
Anton SD, Morrison CD, Cefalu WT (2008) Effects of chromium picolinate on food intake and satiety. Diabetes Technol Ther 10(5):405–412
CAS PubMed PubMed Central Article Google Scholar
-
Arciero PJ, Gardner AW, Calles-Escandon J, Benowitz NL, Poehlman ET (2007) Effects of caffeine ingestion on NE kinetics, fat oxidation, and energy expenditure in younger and older men. Am J Physiol 268:1192–1198
Google Scholar
-
Baglioni S, Cantini G, Poli G, Francalanci M, Squecco R, Di Franco A et al (2012) Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLOS One 7(5):e36569. https://doi.org/10.1371/journal.pone.0036569
ADS CAS Article PubMed PubMed Central Google Scholar
-
Banni S (2012) Conjugated linoleic acid metabolism. Curr Opin. 13(3):261–266
Google Scholar
-
Belza A, Toubro S, Astrup A (2014) The effect of caffeine, green tea and tyrosine on thermogenesis and energy intake. Eur J Clin Nutr 63(1):57–64 Epub 2007 Sep 19
Article CAS Google Scholar
-
Berdanier CR, Gorny JR, Joussif AE (2000) Advanced Nutrition:Macronutrients, 2nd edn. CRC Press, Boca Raton
Google Scholar
-
Bes-Rastrollo M, Sabate J, Gomez-Gracia E, Alonso A, Martinez JA, Martinez-Gonzalez MA (2017) Nut consumption and weight gain in a Mediterranean cohort: The sun study. Obesity 15(1):107–116
Article Google Scholar
-
Biesiekierski JR (2017) What is gluten? J Gastroenterol Hepatol 32(1):78–81. https://doi.org/10.1111/jgh.13703.%20PMID%2028244676
CAS Article PubMed Google Scholar
-
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013) Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev 22(16):2298–2314. https://doi.org/10.1089/scd.2012.0647 PMC 3730538 . PMID 23517218
CAS Article PubMed PubMed Central Google Scholar
-
Bland J, Lyon M, Jones DS (2006) Clinical approaches to detoxification and biotransformation. J Med Assoc 5:34–45
Google Scholar
-
Blumenfeld NR, Kang HJ, Fenzl A, Song Z, Chung JJ, Singh R, Johnson R, Karakecili A, Feranil JB, Rossen NS, Zhang V, Jaggi S, McCarty B, Bessler S, Schwartz GJ, Grant R, Korner J, Kiefer FW, Gillette BM, Samuel SK (2018) A direct tissue-grafting approach to increasing endogenous brown fat. Sci Rep 8(1). https://doi.org/10.1038/s41598-018-25866-y
-
Bobyleva V, Bellei M, Kneer N, Lardy H (2007) The effects of the ergosteroid 7-oxo-dehydroepiandrosterone on mitochondrial membrane potential: possible relationship to thermogenesis. Arch Biochem Biophys 341(1):122–128
Article Google Scholar
-
Boirie M, Dangin Y, Guillet C, Beaufrere B (2011) Influence of the protein digestion rate on protein turnover in young and elderly subjects. J Nutr 132(10):3228S–3233S
Google Scholar
-
Bredsdorff L, Wedebye EB, Nikolov NG, Hallas-Moller T, Pilegaard K (2015) Raspberry ketone in food supplements–high intake, few toxicity data–a cause for safety concern? Regul Toxicol Pharmacol 73:196–200
CAS PubMed Article Google Scholar
-
Brenot F, Abenhaim L, Moride Y, Rich S, Benichou J, Kurz X et al (2015) Appetite-suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med 335:609
Google Scholar
-
Brown JC, Harhay MO, Harhay MN (2017) Anthropometrically-predicted visceral adipose tissue and mortality among men and women in the third national health and nutrition examination survey (NHANES III). Am J Hum Biol 29(1):181–195. https://doi.org/10.1002/ajhb.22898
Article Google Scholar
-
Brownell KD (2009) Greenwood MR Stellar and Eileen E (2009): The effects of repeated cycles of weight loss and regain in rats. Physiol Behav 38(4):459
Article Google Scholar
-
Bucci LR (2008) Selected herbals and human exercise performance. Am J Clin Nutr 72(2):624S–636S
Article Google Scholar
-
Cabrera C, Artacho R, Giménez R (2006) Beneficial effects of green tea; a review. J Am Coll Nutr 25(2):79–99
CAS PubMed Article Google Scholar
-
Cannon B, Nedergaard J, Nute GR (2018) Developmental biology: neither fat nor flesh. Nature 454(7207):947–958
ADS Article CAS Google Scholar
-
Canoy D (2010) Distribution of body fat and risk of metabolic disorders in man and woman. Curr Opin Endocrinol Diabetes 17:143–149
Article CAS Google Scholar
-
Carmen GY, Víctor SM. Signalling mechanisms regulating lipolysis. Cell Signal. 2006;18(4):401–8. https://doi.org/10.1016/j.cellsig.2005.08.009 Epub 2005 Sep 22. PMID: 16182514
CAS PubMed Article Google Scholar
-
Chen M, Pan A, Malik VS, Hu FB (2012) Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. Am J Clin Nutr 96(8):735–747
CAS PubMed PubMed Central Article Google Scholar
-
Christensen R, Lorenzen JK, Svith CR, Bartels EM, Melanson EL, Saris WH et al (2009) Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev 10(2):475–486
CAS PubMed Article Google Scholar
-
Cimolai N, Cimolai T, Kessel J (2011) Yohimbine use for physical enhancement and its potential toxicity. J Diet Suppl 8:346–354
CAS PubMed Article Google Scholar
-
Clapham JC, Arch JR (2007) Thermogenic and metabolic antiobesity drugs: rationale and opportunities. Diabetes Obes Metab 9:259–275
CAS PubMed Article PubMed Central Google Scholar
-
Coelho M, Oliveira T, Fernandes R (2013) Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 9(2):191–200. https://doi.org/10.5114/aoms.2013.33181
CAS Article PubMed PubMed Central Google Scholar
-
Cohen PA, Wang YH, Maller G, DeSouza R, Khan IA (2016) Pharmaceutical quantities of yohimbine found in dietary supplements. Drug Test Anal 8:357–369
CAS PubMed Article Google Scholar
-
Cotten BM, Diamond SA, Banh T, Hsiao YH, Cole RM, Li J et al (2017) Raspberry ketone fails to reduce adiposity beyond decreasing food intake in C57BL/6 mice fed a high-fat diet. Food Funct 8:1512–1518
CAS PubMed Article Google Scholar
-
Coyle LP, Patrick JR (2013) Abete GS (2013): Beneficial facts on Food. J Med Food 35(5):13–19
Google Scholar
-
Delbeke FT, Van Eenoo P, Van Thuyne W, Desmet N (2002) Prohormones and sport. J Steroid Biochem Mol Biol 83(1–5):245–251
CAS PubMed Article Google Scholar
-
Demling RH (2009) Effect of a hypocaloric diet, increased protein intake and resistance training on lean mass gains and fat mass loss in overweight police officers. Ann Nutr Metab 44(1):21–29
Article Google Scholar
-
Denker T, Joel R, Bland J (2012) The world on a plate, 4th edn. Nebraska: Nebraska Press
-
Dennis EA, Dengo AL, Comber DL et al (2010) Water consumption increases weight loss during a hypo caloric diet intervention in middle-aged and older adults. Obesity 18(2):300–307. https://doi.org/10.1038/oby.2009.235
Article PubMed Google Scholar
-
Dhaliwal SS, Welborn TA (2009) Central obesity and multivariable cardiovascular risk as assessed by the Framingham prediction scores. Am J Cardiol 103(10):1403–1407. https://doi.org/10.1016/j.amjcard.2008.12.048
Article PubMed Google Scholar
-
Din MU, Saari T, Raiko J, Kudomi N, Maurer SF, Lahesmaa M, Tobias Fromme T, Amri EZ, Klingenspor M, Solin O, Nuutila P, Virtanen KA (2018) Postprandial oxidative metabolism of human brown fat indicates thermogenesis. Cell Metab 28(2):207. https://doi.org/10.1016/j.cmet.2018.05.020
CAS Article Google Scholar
-
Divoux A, Drolet R, Clement A (2011) Architecture and extracellular matrix of adipose tissue. Obes Rev 12(35):494–503
Article Google Scholar
-
Dubnov-Raz G, Constantini NW, Yariv H, Nice S, Shapira N (2011) Influence of water drinking on resting energy expenditure in overweight children. Int J Obes 35(10):1295–1300. https://doi.org/10.1038/ijo.2011.130
CAS Article Google Scholar
-
Dulloo AG, Geissler CA, Horton T, Collins A, Miller DS (2015) Normal caffeine consumption: influence on thermogenesis and daily energy expenditure in lean and postobese human volunteers. Am J Clin Nutr 49(1):44–50
Article Google Scholar
-
Dulloo AG, Geissler GA, Kangas AJ (2009) Normal caffeine consumption: influence on thermogenesis and daily energy expenditure in lean and post obese human volunteers. Am J Clin Nutr 49(1):44–50
Article Google Scholar
-
Duvernoy CS (2011) The health risks of yoyo dieting. J Med Assoc 15(272):1169
Google Scholar
-
Earthman CP, Beckman LM, Masodkar K, Sibley SD (2012) The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond) 36:387–396
CAS Article Google Scholar
-
Eckel SE, Dolinkov MA, Dost IK, Lacinov ZE, Michalsk YD, Haluz DW, Kasalick YM (2005) The endocrine profile of subcutaneous and visceral adipose tissue of obese patients. Mol Cell Endocrinol 28(17):456–475
Google Scholar
-
Enerbäck S (2009) The origins of brown adipose tissue. N Engl J Med 360(19):2021–2023
PubMed Article Google Scholar
-
Eric E, Berg DC (2010) The 7 principles of fat burning, 1st edn. Blackwell Science, Oxford
Google Scholar
-
Farrell DJ, Bower L, Speedy DB (2013) Fatal water intoxication. J Clin Path 56(10):803–804. https://doi.org/10.1136/jcp.56.10.803-a
Article Google Scholar
-
Fenzl A, Kiefer FW (2014) Brown adipose tissue and thermogenesis. Hormone Mol Biol Clin Investig 19(1):25–37. https://doi.org/10.1515/hmbci-2014-0022 PMID 25390014
CAS Article Google Scholar
-
Fomous CM, Costello RB, Coates PM (2002) Symposium: conference on the science and policy of performance-enhancing products. 34(10):1685–1690
-
Fu C, Jiang Y, Guo J, Su Z (2016) Natural products with anti-obesity effects and different mechanisms of action. J Agric Food Chem 64:9571–9585
CAS PubMed Article Google Scholar
-
Gades MD, Stern JS, Walter AH (2002) Chitosan supplementation does not affect fat absorption in healthy males fed a high-fat diet, a pilot study. Int J Obes Relat Metab Disord 26(1):119–122
CAS PubMed Article Google Scholar
-
Galitzky J, Rivière D, Tran MA, Montastruc JL, Berlan M (1990). Pharmacodynamic effects of chronic yohimbine treatment in healthy volunteers. Eur J Clin Pharmacol. 39(5):447–51. https://doi.org/10.1007/bf00280934
-
Gannon MC, Nuttall FQ. Effect of a high-protein diet on ghrelin, glucagon, and insulin-like growth factor-I in obese subjects. Metabolism. 2011 Sep;60(9):1300-1311. doi: https://doi.org/10.1016/j.metabol.2011.01.016. Epub 2011 Mar 15.
CAS PubMed Article Google Scholar
-
Gittleman AL (2010) The fat flush diet plan review, 3rd edn. Barry Seaars.Mc Groaw-Hill
-
Gittleman AL (2011) Fat flush foods, 4th edn. California: Seasars B.Mc Groaw-Hill
-
Gittleman AL (2012) Fat flush for life: A strategy to achieving weight-loss goals, 5th edn. California: Seasars B.Mc Groaw-Hill
-
Gray JA, Berger M, Roth BL (2016) The expanded biology of serotonin. Annu Rev Med 60:355–366
Google Scholar
-
Greer F, Friars D, Graham TE (2000) Comparison of caffeine, theophylline ingestion: exercise metabolism and endurance. J Appl Physiol 89(5):1837–1844
CAS PubMed Article Google Scholar
-
Grier PT (2007) Hyponatremia "water intoxication", 4th edn. Philadelphia: Lippincott Williams & Wilkins
-
Guerre M, Millo K (2011) Adipose tissue hormones. J Endocrinol Invest 25(10):855–861
Article Google Scholar
-
Guo L, Gurda GT, Lee SH, Molkentin JD, Williams JA (2016) Cholecystokinin activates pancreatic calcineurin-NFAT signaling in vitro and in vivo. Mol Biol Cell 19(1):198–206
Google Scholar
-
Ha E, Zemel MB (2011) Functional properties of whey, whey components, and essential amino acids: mechanisms underlying health benefits for active people (review). J Nutr Biochem 14(5):251–258
Article CAS Google Scholar
-
Haller CA, Anderson IB, Kim SY, Blanc PD (2012) An evaluation of selected herbal. Adverse Drug React Toxicol Rev 21(3):143–150
Article Google Scholar
-
Harms M, Seale P, Pezeshkian S. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013. 19 (10): 1252–1263. doi:https://doi.org/10.1038/nm.3361. PMID 24100998
CAS PubMed Article Google Scholar
-
Harris RB (2011) Leptin-much more than a satiety signal. Ann Rev Nutr 21(6):591–600.0
MathSciNet Google Scholar
-
Hofman Z, Smeets R, Verlaan G, Lugt R, Verstappen PA (2008) The effect of bovine colostrum supplementation on exercise performance in elite field hockey players. Int J Sport Nutr Exerc Metab 12(4):461–469
Article Google Scholar
-
Holm C (2009) Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans 31(6):1120–1124
Article Google Scholar
-
Hooper EF, Maglione M, Mojica WA, Suttorp MJ, Rhodes SL, Jungvig L (2015) Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev 10(6):CD011737
Google Scholar
-
Hsueh WA, Avula B, Pawar RS (2013) Major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab 17(3):411–422
PubMed PubMed Central Article CAS Google Scholar
-
Hursel R, Viechtbauer W, Westerterp-Plantenga MS (2016) The effects of green tea on weight loss and weight maintenance: a meta-analysis. Int J Obes (Lond) 33(9):956–961. https://doi.org/10.1038/ijo.2009.135
CAS Article Google Scholar
-
Imbeault P, Pelletier C, Tremblay A (2016) Energy balance and pollution by organochlorines and polychlorinated biphenyl. 4(1):17–24
-
Inagaki T, Sakai J, Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol. 2016.17 (8):480–495. doi:https://doi.org/10.1038/nrm.2016.62. PMC 4956538 .PMID 27251423.
CAS PubMed PubMed Central Article Google Scholar
-
Ivy JL (2004) Effect of Pyruvate and dihydroxyactetone on metabolism and aerobic endurance capacity. Med Sci Sports Exerc 30(6):837–843
Google Scholar
-
Jeukendrup AE, Randell R (2014) Fat burners: dietary supplements for weight loss. Obes Rev 12(10):841–851. https://doi.org/10.1111/j.1467-789X.2011.00908.x
CAS Article Google Scholar
-
Jeukendrup AE, Randell RE, Coates PM (2016) Fat burners: nutrition supplements that increase fat metabolism. Obes Rev 12(10):841–855
Article CAS Google Scholar
-
Johnson R, Bryant S, Huntley AL (2012) Green tea and green tea in health. J Am sci 23(7):81–95
Google Scholar
-
Jones OA, Maguire ML, Griffin JL (2008) Environmental pollution and diabetes: a neglected association. Lancet 26(37):287–288
Article Google Scholar
-
Julkunen R, Janatuinen E, Kosma M, Mäki M (2012) a comparison of diets with and without oats in adults with celiac disease. Gut 50(3):332–335
Google Scholar
-
Kahn SE, Hull RL, Utzschneider KM (2016) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444(7121):840–846
ADS Article CAS Google Scholar
-
Karastergiou K, Smith SR, Greenberg AR, Fried SK (2012) Sex differences in human adipose tissues – the biology of pear shape. Biol Sex Differ 3:13. https://doi.org/10.1186/2042-6410-3-13
Article PubMed PubMed Central Google Scholar
-
Karst H, Steiniger J, Noack R, Steglich H (2010) Diet-induced thermogenesis in man: thermic effects of single protein, carbohydrates and fats depending on their energy amount. Ann Nutr Metab 28:245–252
Article Google Scholar
-
Kelly TF, Kapoor NK, Lieberman DZ (2009) The use of triiodothyronine as an augmentation agent in treatment-resistant bipolar II and bipolar disorder NOS. J Affect Disord 116(3):222–226
CAS PubMed Article Google Scholar
-
Kennedy A, Martinez K, Schmidt S, Mandrup S, LaPoint K, McIntosh M (2011) Antiobesity mechanisms of action of conjugated linoleic acid. J Nutr Biochem 21(3):171–179. https://doi.org/10.1016/j.jnutbio.2009.08.003
CAS Article Google Scholar
-
Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89(6):2548–2556
CAS PubMed Article Google Scholar
-
Kersten S (2001) Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep 2(4):282–286. https://doi.org/10.1093/embo-reports/kve071 PMC 1083868 . PMID 11306547
CAS Article PubMed PubMed Central Google Scholar
-
Kim BH, Lumor SE, Akoh CC (2014) Trans-free margarines prepared with canola oil/palm stearin/palm kernel oil-based structured lipids. J Agric Food Chem 56(17):8195–8205
Article CAS Google Scholar
-
King MW (2012) Structure and function of hormones: growth hormone. Clin Endocrinol 65(4):413–422
Google Scholar
-
Kissig M, Shapira SN, Seale P (2016) Snap shot: brown and beige adipose thermogenesis. Cell 166(1):258–258.e1. https://doi.org/10.1016/j.cell.2016.06.038
CAS Article PubMed PubMed Central Google Scholar
-
Klein AV, Kiat H (2015) Detox diets for toxin elimination and weight management: a critical review of the evidence. J Hum Nutr Diet. 28(6):675–686. https://doi.org/10.1111/jhn.12286 Epub 2014 Dec 18
CAS Article PubMed Google Scholar
-
Klein S, Peters J, Holland B. Wolfe R.Effect of short- and long-term beta-adrenergic blockade on lipolysis during fasting in humans. Am J Physiol. 2006. 257: E65–E73.
CAS Article Google Scholar
-
La Merrill M, Emond C, Kim MJ, Antignac JP, Le Bizec B, Clément K, Birnbaum LS, Barouki R (2013) Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ Health Perspect 121(2):162–169. https://doi.org/10.1289/ehp.1205485
CAS Article PubMed Google Scholar
-
Lalchandani SG, Lei L, Zheng W, Suni MM, Moore BM, Liggett SB, Miller DD, Feller DR (2002) Yohimbine dimers exhibiting selectivity for the human alpha 2C-adrenoceptor subtype. J Pharmacol Exp Ther. 303(3):979–84. https://doi.org/10.1124/jpet.102.039057
CAS PubMed Article Google Scholar
-
Lambert JD, Sang S, Yang CS (2007) Possible controversy over dietary polyphenols: benefits vs risks. Chem Res Toxicol 20(4):583–585
CAS PubMed Article Google Scholar
-
Lardy H, Partridge B, Kneer N, Wei Y (2007) Ergosteroids: induction of thermogenic enzymes in liver of rats treated with steroids derived from dehydroepiandrosterone. Proc Natl Acad Sci 92(14):6617–6619
ADS Article Google Scholar
-
Lenz TL, Hamilton WR, Ernst E (2013) Supplemental products used for weight loss. J Am Pharm Assoc 44:59–67
Article Google Scholar
-
Leonard ST, Worrel ME, Gurkovskaya OV, Lewis PB, Winsauer PJ (2014) Effects of 7-keto dehydroepiandrosterone on voluntary ethanol intake in male rats. Alcohol 45(4):349–354
Google Scholar
-
Leonard WR (2008) Food for thought: dietary change was a driving force in human evolution. Sci Am 287(6):106–115
Article Google Scholar
-
Li T (2018) Vegetables and fruits: nutritional and therapeutic values. United States: CRC Press, pp 1–2 ISBN 978-1-4200-6873-3
-
Lim SS, Vos-Theo F, Abraham D, Danaei G, Shibuya K, Adair-Rohani H et al (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study. Lancet 380(9859):2224–2260. https://doi.org/10.1016/S0140-6736(12)61766-8
Article Google Scholar
-
Lyon CJ, Law RE, Hsueh WA (2003) Newly discovered endocrine functions of white adipose tissue: possible relevance in obesity-related diseases. Endocrinol 144:3765–3773
Article CAS Google Scholar
-
Lyon M, Bland J, Jones DS (2016) Clinical approaches to detoxification and biotransformation. J Med Assoc 5:34–45
Google Scholar
-
MacDonald E, Kobilka BK, Scheinin M (1997) Gene targeting--homing in on alpha 2-adrenoceptor-subtype function. Trends Pharmacol Sci 1997;18(6):211–9. https://doi.org/10.1016/s0165-6147(97)01063-8
CAS PubMed Article Google Scholar
-
Madgula VL, Avula B, Pawar RS (2008) In vitro metabolic stability and intestinal transport of P57 from Hoodia gordonii. An overview of the clinical evidence. Planta Medica 73(4):280
Google Scholar
-
Mallard SR, Howe AS, Houghton LA (2016) Vitamin D status and weight loss: a systematic review and meta-analysis of randomized and nonrandomized controlled weight-loss trials. Am J Clin Nutr 104:1151–1159
CAS PubMed Article Google Scholar
-
Manore M, Champaign IL, Thompson J (2011) Regulation of fatty acid oxidation in skeletal muscle. Annual Rev Nutr 19:463–484
Google Scholar
-
Manore MM (2015) Dietary supplements for improving body composition and reducing body weight: where is the evidence? Int J Sport Nutr Exerc Metab 22:139–154
Article Google Scholar
-
Mehta T, Smith DL Jr, Muhammad J, Casazza K (2014) Impact of weight cycling on risk of morbidity and mortality. Obes Rev 15(11):870–881. https://doi.org/10.1111/obr.12222
CAS Article PubMed PubMed Central Google Scholar
-
Millan MJ, Mannoury CC, Chanrion B (2012) The role of serotonin in eating disorders. J Pharm Exp Ther 340(3):750–764
CAS Article Google Scholar
-
Montama JP, Coutre IL, Conner KS (2010) In: Berg JM (ed) Fat detection, taste, texture and post ingestive effects, 3rd edn. Springer, New York
-
Muckelbauer R, Sarganas G, Grüneis A, Müller-Nordhorn J (2013) Association between water consumption and body weight outcomes: a systematic review. Am J Clin Nutr 98(2):282–299. https://doi.org/10.3945/ajcn.112.055061
CAS Article PubMed Google Scholar
-
Mudryj AN, Yu N, Aukema HM (2014) Nutritional and health benefits of pulses. Appl Physiol Nutr Metab 39(11):1197–1204. https://doi.org/10.1139/apnm-2013-0557.%20PMID%2025061763
CAS Article PubMed Google Scholar
-
Mussolino ME, Ingram DD, Boirit SE (2010) Weight loss from maximum body weight and mortality: the Third National Health and Nutrition Examination Survey Linked Mortality File. Int J Obes 34:1044–1050
Article Google Scholar
-
Naber AH, Laflamme DP, Hannah SS (2015) Increased protein metabolism promotes fat loss and reduces loss of lean body mass during weight loss. Intern J Appl Res Vet Med 3(2):62–79
Google Scholar
-
Naber AH, Wanten GJ, Boirit SE (2014) Cellular and physiological effects of medium-chain triglycerides. Mini Rev Med Chem 4(8):847–857
Google Scholar
-
Nagai M, Komiya H, Mori Y, Ohta T, Kasahara Y, Ikeda Y (2015) Estimating visceral fat area by multi-frequency bioelectrical impedance. Diabetes Care 33(5):1077–1079. https://doi.org/10.2337/dc09-1099
Article Google Scholar
-
Nawrot P, Jordan S, Leonard S (2013) Effects of caffeine on human health. Food Addit Contam 20(1):1–30
Article Google Scholar
-
Naz A, Butt MS, Sultan MT, MMN Q, Niaz RS (2014) Watermelon lycopene and allied health claims. EXCLI J 13:650–660
PubMed PubMed Central Google Scholar
-
Nordqvist C, Gesta S, Tseng YH, Kahn CR (2012) High prevalence of brown adipose tissue in adult humans. J Clin Endocrinol Metab 96(8):2450–2455
-
Onakpoya I, Hunt K, Wider B, Ernst E (2014) Pyruvate supplementation for weight loss: a systematic review and meta-analysis of randomized clinical trials. Crit Rev Food Sci Nutr 54:17–23
CAS PubMed Article Google Scholar
-
Onakpoya I, Posadzki P, Ernst E (2013) Chromium supplementation in overweight and obesity: a systematic review and meta-analysis of randomized clinical trials. Obes Rev 14:496–507
CAS PubMed Article Google Scholar
-
Onakpoya IJ, Posadzki PP, Watson LK, Davies LA, Ernst E (2012) The efficacy of long-term conjugated linoleic acid (CLA) supplementation on body composition in overweight and obese individuals: a systematic review and meta-analysis of randomized clinical trials. Eur J Nutr 51(2):127–134. https://doi.org/10.1007/s00394-011-0253-9
CAS Article PubMed Google Scholar
-
Parikh SJ, Yanovski JA, Sibley SD (2003) Calcium intake and adiposity. Am J Clin Nutr 77:281–287
CAS PubMed Article Google Scholar
-
Park K (2010) Raspberry ketone increases both lipolysis and fatty acid oxidation in 3 T3-L1 adipocytes. Planta Medica 76(15):1654–1658
CAS PubMed Article Google Scholar
-
Parra DK, Abete GS, Crujeiras AE, Goyenechea EB, Martinez JA (2008) Diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. J Hum Nutr Diet 13(6):491–500
Google Scholar
-
Pathak K, Soares MJ, Calton EK, Zhao Y, Hallett J (2014) Vitamin D body weight status: a systematic review and meta-analysis of randomized controlled trials. Obes Rev 15:528–537
CAS PubMed Article Google Scholar
-
Paul CB (2009) Nutrients that speed fat loss and why you should get them in your diet, 18th edn. McGraw-Hill, Boston
Google Scholar
-
Paul GS, Mary LS, Morton MMP, Walter AM, Rhodes FA MSL, Gagné MS (2011) Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA 289:1537–1545
Google Scholar
-
Picco MF (2012) Is colon cleansing a good way to eliminate toxins from your body? Colorectal Dis 11:686 Mayo Clinic, Jacksonville, Fla
Google Scholar
-
Podder K, Kolge S, Benzman L, Mullin G, Cheskin L (2011) Nutraceutical supplements for weight loss: a systemic Review. Nutr Clin Pract 26(50):539–552
Article Google Scholar
-
Pooyandjoo M, Nouhi M, Shab-Bidar S, Djafarian K, Olyaeemanesh A (2016) The effect of (L-)carnitine on weight loss in adults: a systematic review and meta-analysis of randomized controlled trials. Obes Rev 17:970–976
CAS PubMed Article Google Scholar
-
Porter SA, Massaro JM, Hoffmann U, Fox CS (2009) Abdominal subcutaneous adipose tissue: a protective fat depot? Diab Care 32(6):1068–1075
Article Google Scholar
-
Prentice AM (2017) Macronutrients as sources of food energy. New England J Med 8(7):932–939. https://doi.org/10.1079/PHN2005779
Article Google Scholar
-
Rajamohan T, Sandhya VG, Schneider P (2016) Effects of coconut water feeding on lipid metabolism in cholesterol-fed rats. J Med Food 9(3):400–407
Google Scholar
-
Ramdath D, Renwick S, Duncan AM (2016) The role of pulses in the dietary management of diabetes. Can J Diabetes 40(4):355–363. https://doi.org/10.1016/j.jcjd.2016.05.015
Article PubMed Google Scholar
-
Rao N, Spiller HA, Hodges NL, Chounthirath T, Casavant MJ, Kamboj AK et al (2017) An increase in dietary supplement exposures reported to US poison control centers. J Med Toxicol 24(4):56–68
Google Scholar
-
Reiner S, Ambrosio M, Hoffmann C, Lohse MJ (2010) Differential signaling of the endogenous agonists at the beta2-adrenergic receptor. J Biol Chem 285(46):36188–98. https://doi.org/10.1074/jbc.M110.175604
CAS PubMed Article Google Scholar
-
Reuter SE, Evans AM (2012) Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet 51:553–572
CAS PubMed Article Google Scholar
-
Rhoades RA, Tanner GA (2003) Medical Physiology, 2nd edn. Lippincott Williams & Wilkins, Baltimore ISBN 0-7817-1936-4. OCLC 50554808
Google Scholar
-
Rios-Hoyo A, Salmean G, Nyangono G (2016) New dietary supplements for obesity: what we currently know. Curr Obes Rep 5:262–270
PubMed Article Google Scholar
-
Robergs RA, Keteyian SJ (2013) Fundamentals of exercise physiology: for fitness, performance, and health, 2nd edn. McGraw-Hill Higher Education, Boston
Google Scholar
-
Rosenberg IH, Popkin BM, D'Anci KE (2010) Water-rich food and health. Nutr Rev 68(8):439–458. https://doi.org/10.1111/j.1753-4887.2010.00304.x
Article PubMed PubMed Central Google Scholar
-
Schmitt JA, Coutre IL, Wilkins SE (2015) Food ingredients and cognitive performance. Curr Opin Clin Nutr Metab Care 11:706–710
Google Scholar
-
Schneider P, Sandhya VG, Rajamohan T (2016) Beneficial effects of coconut water feeding on lipid metabolism in cholesterol-fed rats. J Med Food 9(3):400–407
Google Scholar
-
Schwenk TL, Hardy ML, Costley CD (2003) When food becomes a drug: nonanabolic nutritional supplement use in athletes. Am J Sports Med 30(6):907
Article Google Scholar
-
Serna-Saldivar SO (2015) Cereal grains: laboratory reference and procedures Manual. Food Preservation Technology. United Kingdom: Taylor & Francis, p 58 ISBN 978-1-4398-5565-2
-
Shekelle PG, Hardy ML, Morton SC, Maglione M, Mojica WA, Suttorp MJ, Rhodes SL, Jungvig L, Gagne J (2003b) Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance. JAMA 289(12):1537–1545
CAS PubMed Google Scholar
-
Shekelle PG, Hardy ML, Morton SC, Maglione M, Mojica WA, Suttorp MJ et al (2003a) Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis. JAMA 289:1537–1545
CAS PubMed Google Scholar
-
Smith C, Krygsman A, Ogawa H (2014) Hoodia gordonii: to eat, or not to eat. J Ethnopharmacol 155:987–991
PubMed Article Google Scholar
-
Smith IK (2016) The trouble with fat-burner pills. Endocrinol 55:123–143
Google Scholar
-
Soerensen KV, Thorning TK, Astrup A, Kristensen M, Lorenzen JK (2014) Effect of dairy calcium from cheese and milk on fecal fat excretion, blood lipids, and appetite in young men. Am J Clin Nutr 99:984–991
CAS PubMed Article PubMed Central Google Scholar
-
Spadafranca A, Rinelli S, Riva A, Morazzoni P, Magni P, Bertoli S et al (2013) Phaseolus vulgaris extract affects glycometabolic and appetite control in healthy human subjects. Br J Nutr 109:1789–1795
CAS PubMed Article PubMed Central Google Scholar
-
Stookey JD, Constant F, Popkin BM, Gardner CD (2008) Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity 16(11):2481–2488. https://doi.org/10.1038/oby.2008.409
Article PubMed PubMed Central Google Scholar
-
Stunkard AJ, Harris JR, Pederson NL, McClearn GE (2007) Genetic differences in adipose tissue metabolism and regulation. N Engl J Med 322:1483–1487
Article Google Scholar
-
Sulcová J, Hampl R, Hill M, Stárka L, Novácek A (2005) Delayed effects of short-term transdermal application of 7-oxo-dehydroepiandrosterone on its metabolites, some hormonal steroids and relevant proteohormones in healthy male volunteers. Clin Chem Lab Med 43(2):221–227
PubMed Article CAS PubMed Central Google Scholar
-
Terry L (2011) Health-promoting properties of fruits and vegetables. United Kingdom: CABI, pp 2–4 ISBN 978-1-84593-529-0
-
Tian H, Guo X, Wang X, He Z, Sun R, Ge S et al (2016) Chromium picolinate supplementation for overweight or obese adults. Cochrane Database Syst Rev. 11:CD010063
Google Scholar
-
Trayhurn PY, Wood NS (2004) Adipokines: inflammation and the pleiotropic role of white adipose. J Clin Sci 92(3):347–355
CAS Google Scholar
-
Turcotte LP (2000) Muscle fatty acid up take during exercise: possible mechanism. Exerc Sport Sci Rev 28(1):4–9
CAS PubMed Google Scholar
-
Uckoo RM, Jayaprakasha GK, Nelson SD, Patil BS (2011) Rapid simultaneous determination of amines and organic acids in citrus using high-performance liquid chromatography. Talanta 83:948–954
CAS PubMed Article Google Scholar
-
van Avesaat M, Troost FJ, Westerterp-Plantenga MS, Helyes Z, Le Roux CW, Dekker J et al (2016) Capsaicin-induced satiety is associated with gastrointestinal distress but not with the release of satiety hormones. Am J Clin Nutr 103:305–313
PubMed Article CAS Google Scholar
-
Vanhala M, Saltevo J, Soininen P, Kautiainen H, Kangas AJ, Ala-Korpela M, Mäntyselkä P (2012) Serum omega-6 polyunsaturated fatty acids and the metabolic syndrome: a longitudinal population-based cohort study. Plant Biotechnol J 176(3):253–260
Google Scholar
-
Vij VA, Joshi AS (2013) Effect of water-induced thermogenesis on body weight, body mass index and body composition of overweight subjects. J Clin Diagn Res 7(9):1894–1896. https://doi.org/10.7860/JCDR/2013/5862.3344
Article PubMed PubMed Central Google Scholar
-
Villarroel P, Villalobos E, Reyes M, Cifuentes M (2014) Calcium, obesity, and the role of the calcium-sensing receptor. Nutr Rev 72:627–637
PubMed Article Google Scholar
-
Vincent JB, Sack DA, Roffman M, Finch M, Komorowski JR (2003) The potential value and toxicity of chromium picolinate as a nutritional supplement, weight loss agent and muscle development agent. Sports Med 33(3):213–230
PubMed Article Google Scholar
-
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525
CAS PubMed Article Google Scholar
-
Wardlaw DF, Gordon ME, Kessel MJ (2002) Perspectives in Nutrition, 5th edn. McGraw-Hill, Boston
Google Scholar
-
Westerterp-Plantenga MS (2010) Green tea catechins, caffeine and body-weight regulation. Physiol Behav 100(1):42–46
CAS PubMed Article Google Scholar
-
Westerterp-Plantenga MS, Lejeune MP, Kovacs EM (2005) Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res 13(7):1195–1204
CAS PubMed Article PubMed Central Google Scholar
-
Whelan AM, Jurgens TM, Szeto V (2010) Case report. Efficacy of hoodia for weight loss: is there evidence to support the efficacy claims? J Clin Pharm Ther 35:609–612
CAS PubMed Article Google Scholar
-
Whingham LD, Watras CA, Scholler DA (2007) Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr 85(5):1203–1200
Article Google Scholar
-
Whitehead A, Beck EJ, Tosh S, Wolever TM (2014) Cholesterol-lowering effects of oat β-glucan: a meta-analysis of randomized controlled trials. Am J Clin Nutr 100(6):1413–1421. https://doi.org/10.3945/ajcn.114.086108
CAS Article PubMed PubMed Central Google Scholar
-
Whiting S, Derbyshire EJ, Tiwari B (2014) Could capsaicinoids help to support weight management? A systematic review and meta-analysis of energy intake data. Appetite 73:183–188
CAS PubMed Article Google Scholar
-
Wilson JE, Thomas DR, Margaret MG, Morley RT (2011) Weight reduction review. Am J of Clin Nutr 83(4):735–743
Google Scholar
-
Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richard RD (2008) Fat deposition, fatty acid composition and meat quality: a review. Meat Sci 78(4):343–358
CAS PubMed Article Google Scholar
-
Yang L, Ying C, Zhen Y (2004) Stability of conjugated linoleic acid isomers in egg yolk lipids during frying. Food Chem 86(4):531–535
CAS Article Google Scholar
-
Yanovski JA, Parikh SJ, Yanoff LB, Denkinger BI, Calis KA, Reynolds JC et al (2009) Effects of calcium supplementation on body weight and adiposity in overweight and obese adults: a randomized trial. Ann Intern Med 15(6):821–829
Article Google Scholar
-
Ylitalo R, Lehtinen S, Wuolijoki E, Ylitalo P, Lehtimaki T (2012) Cholesterol-lowering properties and safety of chitosan. Arzneimittelforschung 52(1):1–7
Google Scholar
-
Young L, Anderson J, Roach J (2010) The fiber 35 diet review, 3rd edn. United States: Angie K.Mc Groaw- Hill
-
Zahorska MB, Krotkiewski M, Olszanecka M, Zurakowski A (2011) Effect of chitosan in complex management of obesity. Pol Merkuriusz Lek 13(74):129–132
Google Scholar
-
Zenk JL, Frestedt JL, Kuskowski MA (2012) HUM5007, a novel combination of thermogenic compounds, and 3-acetyl-7-oxo-dehydroepiandrosterone: each increases the resting metabolic rate of overweight adults. J Nutr Biochem 18(9):629–634
Article CAS Google Scholar
Download references
Acknowledgements
The authors would like to thank the National Research Centre where they work.
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Reprints and Permissions
About this article
Cite this article
El-Zayat, S.R., Sibaii, H. & El-Shamy, K.A. Physiological process of fat loss. Bull Natl Res Cent 43, 208 (2019). https://doi.org/10.1186/s42269-019-0238-z
Download citation
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s42269-019-0238-z
Keywords
- Adipose tissue
- Fat loss
- Fat burners
- Fat flush diet
Trouble Metabolizing Fat What Diet
Source: https://bnrc.springeropen.com/articles/10.1186/s42269-019-0238-z